Περιγραφή
Intended use:
For in vitro diagnsotic use. The RIDA®QUICK SARS-CoV-2 Antigen test is a manual immunochromatographic rapid detection test for the qualitative detection of SARS-CoV-2-specific antigens in human respiratory samples from persons with a reasonable suspicion and/or symptoms of SARS-CoV-2 infection as well as from asymptomatic persons.
The RIDA®QUICK SARS-CoV-2 Antigen rapid test is a diagnostic aid to detect and/or rule out a respiratory tract infection with SARS-CoV-2 in connection with other clinical and laboratory findings.
Negative results do not rule out infection with SARS-CoV-2 and should not be used as the sole basis for diagnosis.
The product is intended exclusively for professional use.
General information:
At the end of December 2019 in the Chinese metropolis of Wuhan, numerous cases of pneumonia of unknown cause occurred.1 At the beginning of January, Chinese authorities identified a novel coronavirus (SARS-CoV-2) as the cause of these illnesses.1 The disease caused by SARS-CoV-2 was officially named COVID-19 (“coronavirus disease 2019”) and is transmitted from human to human via droplet infection and aerosols.
The virus spread rapidly across the globe. In response, the WHO declared the disease caused by SARS-CoV-2 a pandemic on 2020-03-11.4
The disease courses are nonspecific and have highly variable degrees of symptoms and severity. The most commonly reported symptoms are cough, fever, and nasal congestion, as well as loss of smell and taste.
At first, only molecular diagnostic technologies, like RT-PCR, were used for the direct identification of the pathogen in throat and nasal swabs, but now antigen detection systems for SARS-CoV-2 are available that have a very good method for detecting SARS-CoV-2 infection in an outbreak situation.
Highlights:
- Official partner of the Federal Ministry of Health for SARS-CoV-2 diagnostics to combat COVID-19
- Included on the list of quality-checked antigen rapid tests by the Paul-Ehrlich-Institut
Features and benefits:
- nasal and throat swab applicable
- high sensitivity and specificity compared to PCR
- results in 20 minutes
- device-independent
- Available in EU/EFTA and selected countries
Videos
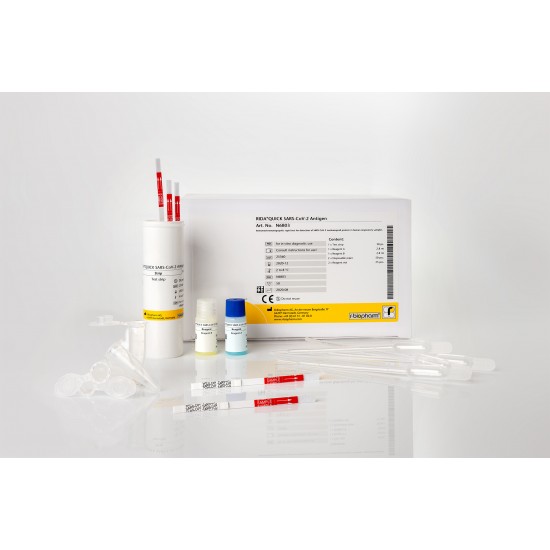
| Art. No. | N6803 |
|---|---|
| Test format | 50 rapid tests |
| Detection limit | 237 TCID50/ml |
| Shelf life | 6 months |
| Sensitivity | 95 % |
| Specificity | 100 % |
| Featured Product | 1 |
| Χαρακτηριστικά | |
| Οδηγίες Χρήσεως |
- Stock: In Stock
- Model: N6803